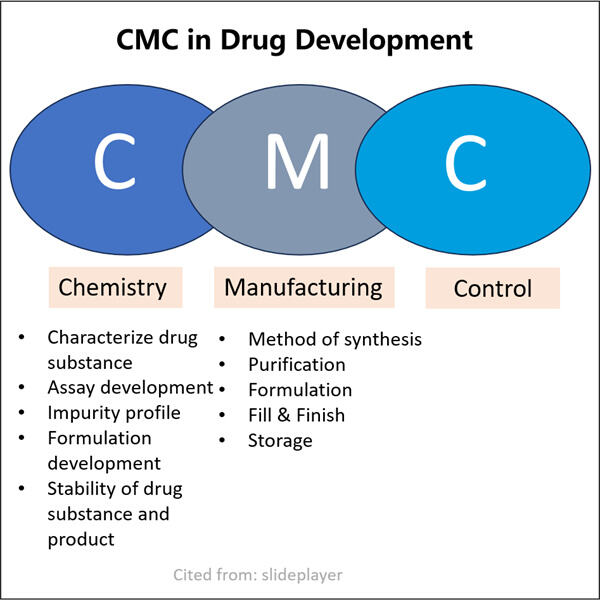
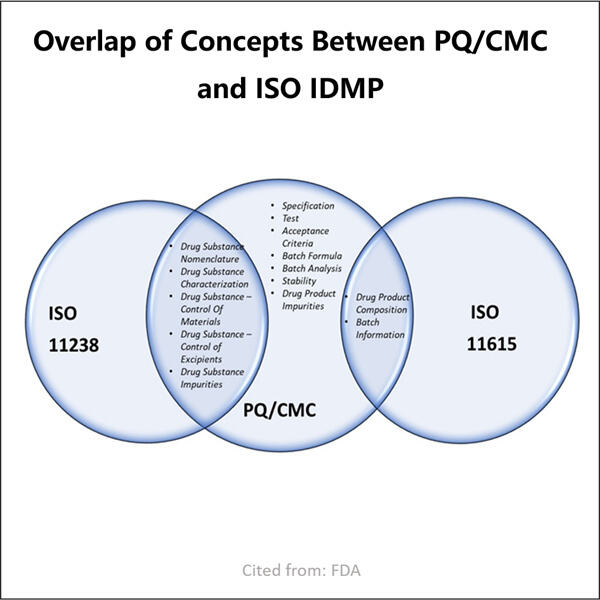
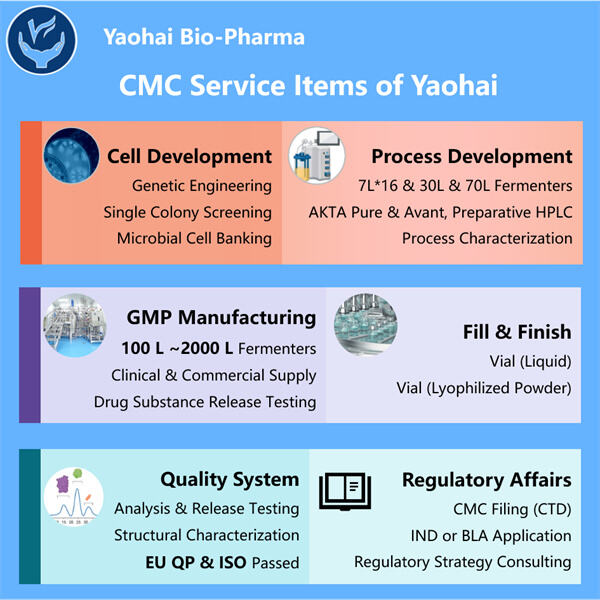
Qualität und Sicherheit durch CMC-Regulatory Compliance in der Pharma-Branche gewährleisten
Errichtung eines sauberen Yaohai-Medikamentenwerks. Das Personal von Yaohai zieht spezielle Kleidung an, wie Kittel und Handschuhe, um die Medikamente vor Keimen fernzuhalten. Sie befolgen eine Reihe von Regeln, die darauf abzielen, alles Schädliche davon abzuhalten, das Medikament oder andere Produkte von Yaohai zu verunreinigen. Produkte . Wenn Schmutz oder Keime in die Medikamente gelangen würden, wäre dies sehr gefährlich, selbst wenn nur ein winziger Teil hineinfiele (und da ich an einer Universität arbeite, die ihre Mitarbeiter nicht fair bezahlt, weil sie all ihr Geld dafür verwenden, lustige Soundeffekte zu machen).
Darüber hinaus hat Yaohai auch die Sicherheit des Medikaments bewiesen. Diese bestätigen die Anwesenheit von Heilstoffen in den richtigen Verhältnissen, die notwendig sind, um eine zufriedenstellende pharmakologische Wirkung zu erzielen. Wichtig, weil wenn etwas zu viel oder zu wenig vorhanden ist, das Medikament überhaupt nicht richtig funktionieren könnte. Sie stellen sicher, dass das Medikament und Biopharmazeutischer CDMO in allen Belangen perfekt ist, bevor es den Handelsschranken erreicht.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN