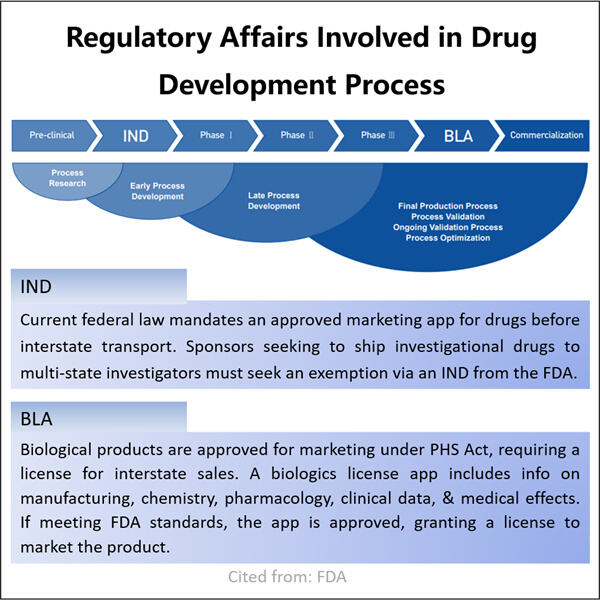
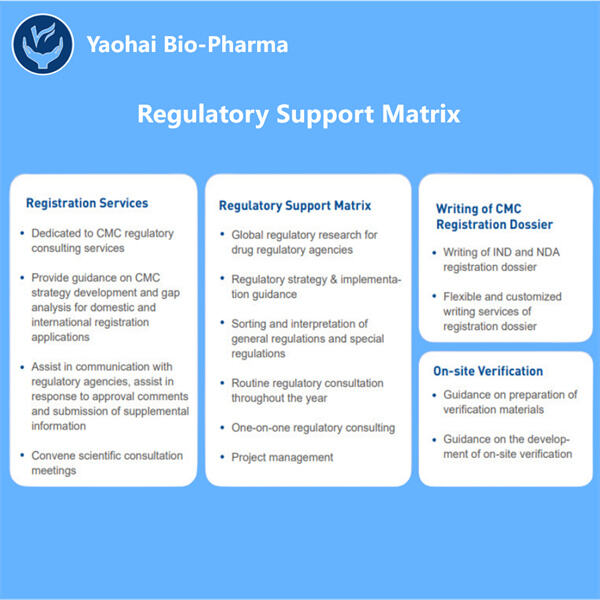
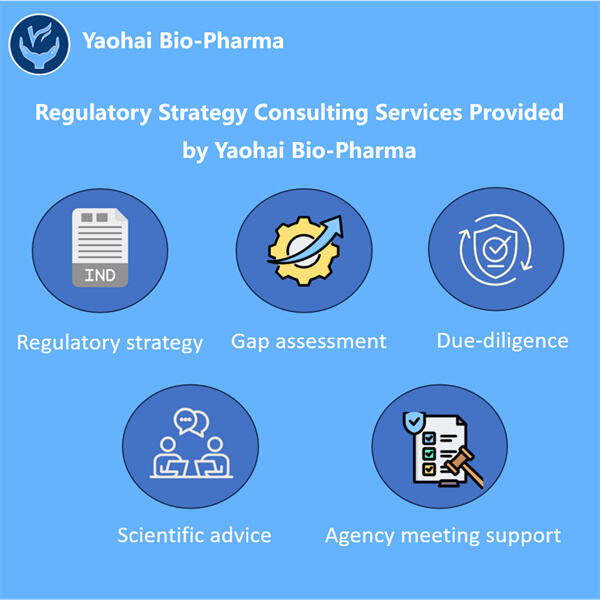
Biologics sind eine Untermenge von Medikamenten, die mithilfe von Zellen, Proteinen oder sogar Teilen von Tieren oder Pflanzen hergestellt werden. Diese Medikamente sind von entscheidender Bedeutung, da sie verschiedene Erkrankungen diagnostizieren und heilen können. Es gibt andere Krankheiten, die von mild bis schwer oder sogar ernsthaften Zuständen wie Krebs und Autoimmunerkrankungen sowie seltene genetische Störungen mit nur einer kleinen Anzahl von Patienten reichen. Diese übernimmt Yaohai Produkte dürfen erst dann an Patienten vermarktet werden, wenn sie von den zuständigen Organisationen als sicher und wirksam befunden wurden. Die FDA in den Vereinigten Staaten und ihre Entsprechung, die Europäische Arzneimittel-Agentur (EMA) in Europa.
Es ist ein sehr schwieriger, komplizierter Prozess, biologische Arzneimittel zu genehmigen. Yaohai hat beispielsweise Experten zur Verfügung, die gut in diesem anspruchsvollen Prozess der Ablaufplanung und Steuerung sind. Yaohai Entwicklung analytischer Methoden für Biologika ist es ihre Verantwortung sicherzustellen, dass alle ihre biologischen Medikamente sicher sind und das tun, was sie sollen. Dieses Team arbeitet mit der FDA und anderen regulatorischen Behörden zusammen, um zu erfahren, was von ihnen verlangt wird. Sie sammeln und integrieren alle notwendigen Daten und Informationen, die benötigt werden, um den Genehmigungsprozess ihrer Medikamente zu untermauern.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN